In research highlighting the intricate battle between viruses and the host immune response, a team of scientists has uncovered how the tegument protein VP22 from pseudorabies virus (PRV) effectively inhibits crucial immune mechanisms. This study sheds light on the virus’s strategy to evade the host’s antiviral defenses, particularly the type I interferon (IFN-I) response, which plays a vital role in fighting off viral infections.
Understanding cGAS Function
At the forefront of the innate immune system is cyclic GMP-AMP synthase (cGAS), a key DNA sensor that initiates antiviral responses. Upon detecting double-stranded DNA (dsDNA), cGAS activates by undergoing oligomerization, forming clusters crucial for its enzymatic activity. This results in the synthesis of 2′3′-cyclic GMP-AMP (cGAMP), which activates the adaptor protein Stimulator of Interferon Genes (STING), thereby triggering a signaling cascade that leads to IFN-I production. However, many DNA viruses, to establish successful infections, have developed mechanisms to evade cGAS activity.
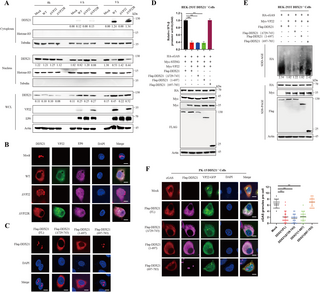
The team’s study reveals that PRV VP22 serves as a viral antagonist of the cGAS pathway by interacting with the host RNA helicase DDX21. The interaction between VP22 and DDX21 not only stabilizes DDX21 but also promotes its translocation from the nucleus to the cytoplasm. This translocation process is critical for VP22’s inhibition of cGAS condensation and activation.
“Our findings indicate that VP22 disrupts the ability of cGAS to form condensates, which are essential for its function,” said lead author Dr. Liu. “By hijacking DDX21, the virus effectively undermines the host’s immune detection capabilities.”
The research demonstrated that in the presence of VP22, cGAMP production significantly decreased, which in turn resulted in reduced activation of IFN-I and impaired antiviral responses. In experiments where the DDX21 gene was knocked out, the VP22-mediated suppression of cGAS activity was reversed, emphasizing DDX21’s pivotal role in this inhibitory process.
In Vivo Effects of VP22
In vivo studies further confirmed the significance of DDX21 in modulating PRV infection. Experiments on mouse embryonic fibroblasts (MEFs) highlighted that PRV strains deficient in VP22 exhibited higher replication rates in cells lacking cGAS. Additionally, mortality rates differed when wild-type and cGAS-deficient mice were infected with the VP22-deficient PRV, showcasing the protective role of cGAS against viral infection.
Implications for Future Research
The significance of these findings extends beyond PRV, offering insights into how other viruses may utilize similar pathways to evade host defenses. The researchers suggest that further exploration of the DDX21-cGAS interaction could yield potential therapeutic targets for antiviral strategies.
“Understanding the mechanisms of viral immune evasion is crucial for developing effective treatments,” Dr. Liu added. “Our study provides a foundational understanding that can lead to new antiviral interventions.”
These findings provide a significant step toward elucidating the complex dynamics between viruses and host immune responses, highlighting the sophisticated tactics employed by PRV to manipulate cellular mechanisms, thus opening avenues for further research in viral pathogenesis and immune modulation.
Research Details
Published as an uncorrected proof in PLoS Pathogens, the study titled “The tegument protein VP22 of pseudorabies virus inhibits cGAS condensation by inducing nuclear-to-cytoplasmic translocation of DDX21” was conducted by a team led by researchers Liu, Cao, Zhang, Yang, Wu, Wang, and others. This research received funding from the National Natural Science Foundation of China, the Natural Science Foundation of Jiangsu Province, and the Jiangsu Independent Innovation Fund Project. All authors declared no competing interests.