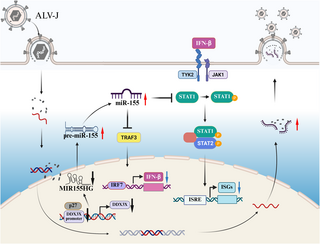
Avian leukosis virus subgroup J (ALV-J) is a significant oncogenic retrovirus that leads to severe health issues in poultry, including immunosuppression and the formation of tumors like myelocytomas. While previous research has established that ALV-J can evade innate immunity, the specific mechanisms behind this evasion have remained elusive. Recent studies reveal that ALV-J enhances the expression of microRNA-155 (miR-155), a key player in the suppression of immune responses, ultimately facilitating viral replication.
In our research, we identified that the p27 viral protein from ALV-J significantly reduces the activity of a cellular protein called DEAD-box helicase 3X (DDX3X). This action diminishes DDX3X expression and promotes the maturation of the MIR155 host gene into active miR-155. The mature miR-155 then targets two crucial proteins, tumor necrosis factor receptor-associated factor 3 (TRAF3) and signal transducer and activator of transcription 1 (STAT1), both of which are essential for the initiation of type I interferon (IFN-I) responses. By silencing TRAF3 or STAT1, researchers found that ALV-J replication, which would normally be inhibited by miR-155, is restored.
This discovery marks a notable advance in understanding the immune evasion tactics employed by retroviruses such as ALV-J, shedding light on their complex pathogenesis.
Author Summary
ALV-J is known for causing considerable economic repercussions in the poultry industry due to its ability to disrupt immune function and induce tumors. Our study illuminates how ALV-J manipulates the host microRNA, miR-155, to escape the body’s antiviral responses. By compromising the function of DDX3X, ALV-J can increase miR-155 levels. This rise in miR-155, in turn, blocks the action of TRAF3 and STAT1—critical regulators in the immune response—thereby suppressing the production of interferons that would typically hinder viral replication. Through this mechanism, the virus effectively lowers its chances of being eradicated by the host immune system.
Introduction
ALV-J represents a rapidly evolving oncogenic retrovirus that poses significant health threats to poultry by inducing immunosuppression and neoplastic diseases. These conditions lead to reduced growth rates, susceptibility to infections, and overall productivity losses in affected flocks. The virus can spread both horizontally and vertically, with vertical transmission linked to more pronounced pathogenic effects, especially during early stages of a chicken’s life.
The ability of ALV-J to evade the host immune response is crucial for its infection and persistence. This evasion is facilitated by the virus’s unique strategies that exploit host factors, such as miRNAs, to counteract antiviral defenses. However, the underlying mechanisms by which ALV-J escapes immune detection remain less well-defined.
miRNA and Immune Evasion
miRNAs are small, non-coding RNAs that regulate gene expression. They play significant roles in both innate and adaptive immune responses, making them intriguing targets for viral immune evasion strategies. For instance, studies have shown that several oncogenic viruses effectively manipulate host miRNA functions to promote their own longevity and pathogenicity.
Our research highlights that while ALV-J induces various miRNAs, including miR-200b-3p, miR-34b-5p, and miR-23b, miR-155 is particularly prominent in the virus’s ability to escape immune responses. Elevated levels of miR-155 were found to block critical components of the host’s antiviral response, particularly affecting IFN production.
Experimental Findings
To explore how ALV-J influences miR-155 levels and subsequent immune suppression, CEFs (chicken embryo fibroblasts) were infected with ALV-J, revealing that infection led to a substantial increase in miR-155 levels. Notably, DDX3X was identified as a significant interacting partner that p27 protein of ALV-J targets to promote miR-155 maturation.
Following this, functional assays demonstrated that increasing miR-155 exerted a negative impact on ALV-J-induced IFN-β production, highlighting the intricate mechanism by which ALV-J manipulates the host immune response. Importantly, DDX3X overexpression was found to reverse the miR-155 induced decrease in TRAF3 and STAT1, confirming that these proteins are key targets for miR-155-mediated immune evasion during viral infections.
Conclusions
The findings establish a novel miR-155-dependent pathway whereby ALV-J sidesteps host immune defenses, ultimately promoting viral replication and persistence. This mechanism illustrates the complex dynamics of host-virus interactions and opens avenues for future research into potential therapeutic targets that could disrupt these viral evasion strategies.
Data Availability
All data supporting this research are provided in the manuscript and supplementary files.
Funding
This study received financial support from several sources, including the Natural Science Foundation of China and the Key Research and Development Program of Shandong Province.
Keywords
- Avian leukosis virus subgroup J
- miR-155
- Innate immunity
- TRAF3
- STAT1
- Viral replication