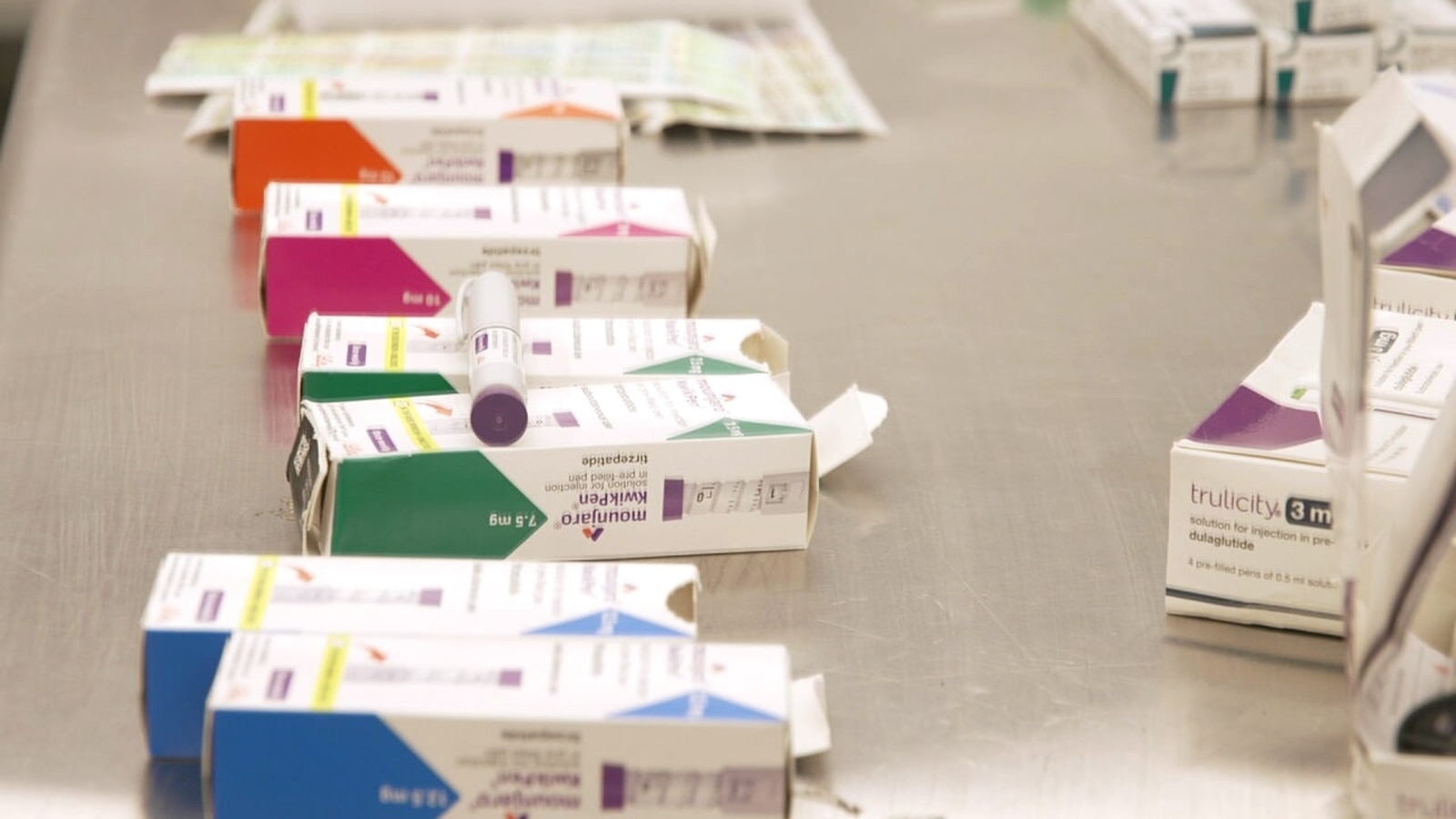
The rising demand for weight loss medications has led to an alarming increase in counterfeit products being sold through online platforms. The U.S. Food and Drug Administration (FDA) has issued warnings regarding unapproved and potentially hazardous versions of popular GLP-1 medications.
Nicole Johnson, a special agent with Homeland Security Investigations, shared her concerns with ABC’s “Nightline” anchor Juju Chang. Johnson stated, “I have absolutely witnessed a surge in counterfeit weight loss drugs recently. Patients are willing to do anything to get their hands on this product, and they’re risking their lives.”
Many individuals are now purchasing these medications from illegal online sellers. A simple internet search unveils numerous alleged vendors, some of which operate in the shadows of the dark web, offering deceitfully branded products like “Fauxzempic” alongside other fake weight loss solutions.
Eric Feinberg, Vice President of content moderation at the Coalition for A Safer Web, emphasized the warning signs to identify counterfeit products. He mentioned that a primary indicator is if a seller states, “No Rx” in their profile.
FDA-approved medications such as Ozempic, Wegovy, Mounjaro, and Zepbound are subject to stringent regulations that include inspections of manufacturing facilities and extensive safety and efficacy studies. According to Feinberg, patients cannot obtain prescription drugs without a legitimate prescription. He urged caution, stating, “The minute you see ‘no prescription needed’ and also the payment processes like bitcoin, Zelle, PayPal — know, it can’t be [authentic].”
Feinberg further noted that many of these counterfeit weight loss drugs are sourced from abroad. When these illicit products enter the United States, they typically pass through major ports, where authorities have seized shipments of illegal GLP-1 drugs, often due to suspicious packaging.
Eric Zizelman, the port director for U.S. Customs and Border Protection at the Port of Cincinnati, reported to ABC News about some confiscated GLP-1 products featuring Spanish labeling. He pointed out that this is a clear sign they are not legitimate products for legal sale in the U.S. Zizelman stated, “Something that’s going to come into the U.S. as a legitimate product is not going to be in Spanish. It has to be in English.”
Zizelman defined drugs made for foreign markets that are then sold in the U.S. as “gray market drugs.” He explained that both gray market and counterfeit drugs are subject to seizure by U.S. Customs and Border Protection agents. He explained, “So gray market are drugs that are typically made for a non-U.S. market and then imported here and sold here, so they’re not approved to be sold inside the United States. They are approved to be sold somewhere else.”
The risks associated with these drugs are significant. Investigative health reporter Katherine Eban, who is also a special correspondent for Vanity Fair, noted, “Once a drug leaves its intended route, really all bets are off — you cannot vouch for the quality of that drug.”
The dangers of counterfeit weight loss drugs extend beyond potential health risks; they undermine legitimate pharmaceutical standards and consumer safety. As demand continues to grow, both consumers and regulatory bodies are urged to remain vigilant against these deceptive practices.