A groundbreaking study reveals that scientists have achieved notable success in reversing Alzheimer’s disease in mouse models by restoring the brain’s vascular system, which is crucial for providing oxygen and nutrients. This significant research was conducted by teams from the Institute for Bioengineering of Catalonia (IBEC) and West China Hospital of Sichuan University (WCHSU), alongside collaborators from the United Kingdom, utilizing innovative nanotechnology.
Researcher Lorena Ruiz Perez, part of the Molecular Bionics group at IBEC, expressed optimism about their findings. She stated, “Our study demonstrated remarkable efficacy in achieving rapid Aβ clearance… restoring healthy function in the blood–brain barrier… leading to a striking reversal of Alzheimer’s pathology.”
This revolutionary approach to treating Alzheimer’s employs nanoparticles not just as vehicles for delivering drugs but as active therapeutic agents known as supramolecular drugs. Rather than focusing on targeting neurons or other brain cells directly, this therapy aims to restore the essential function of the brain’s blood–brain barrier (BBB), often referred to as the “vascular gatekeeper” essential for maintaining a stable brain environment.
“By restoring this ‘gatekeeper,’ we help the brain rebalance itself and make any other therapy work better,” Giuseppe Battaglia, a research professor at IBEC and member of the Catalan Institution for Research and Advanced Studies (ICREA), commented in an interview with Newsweek. He emphasized the need for groundbreaking strategies, saying, “Today, no treatment reliably reverses Alzheimer’s; at best, some slow aspects of decline. Our results point to a new path: fix the barrier that keeps the brain healthy.”
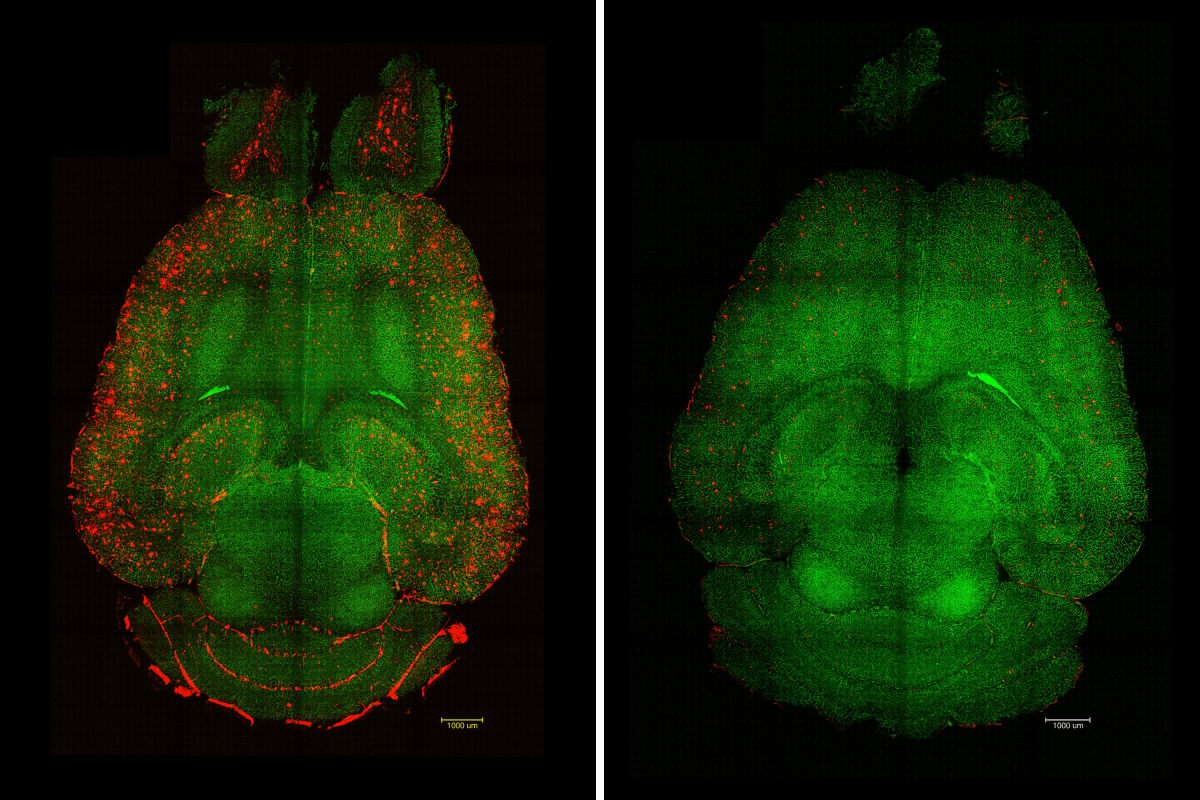
The study underscores the crucial role of vascular health and its intricate connection with neurodegenerative diseases like Alzheimer’s. The researchers showed that by targeting a specific mechanism, harmful “waste proteins” produced in the brain could pass through the BBB, allowing them to be eliminated from the bloodstream effectively.
In Alzheimer’s patients, the buildup of amyloid-beta (Aβ) occurs, disrupting normal neuronal functioning. The study utilized genetically modified mouse models that produce excess Aβ and exhibit substantial cognitive decline reminiscent of Alzheimer’s disease.
“Only one hour after the injection [of the supramolecular drugs], we observed a reduction of 50-60 percent in Aβ amount inside the brain,” described study author Junyang Chen, a researcher at WCHSU and a student at University College London. This rapid response was coupled with a lasting functional recovery observed months later, indicating sustained benefits from the treatment, as Battaglia noted: “No damage or toxicity [was] observed in animals; they tolerate the therapy well.”
In a specific experiment, researchers treated a 12-month-old mouse—comparable to a human aged 60—with the nanoparticles. Six months later, after the mouse reached 18 months of age (analogous to a 90-year-old human), its behavioral and memory assessments revealed a recovery to levels similar to that of healthy mice.
“We think it works like a cascade: when toxic species such as amyloid-beta accumulate, disease progresses. But once the vasculature is able to function again, it starts clearing Aβ and other harmful molecules, allowing the whole system to recover its balance,” Chen elaborated. This suggests a potential mechanism whereby restoring vascular function may enhance brain health.
The nanoparticles function by mimicking the actions of natural molecules interacting with the LRP1 receptor, typically a molecular gatekeeper. Their precision enables these drugs to attach to Aβ, cross the BBB, and activate the clearance of toxic species from the brain, thus revitalizing the vascular system’s waste-removal capabilities.
This remarkable study opens avenues for developing effective clinical treatments targeting the vascular aspects of Alzheimer’s disease, potentially leading to better outcomes for patients. However, further research is crucial to determine how these findings might apply to humans. Battaglia stressed, “If we can safely trigger the same recovery of barrier function in people, we expect improved brain housekeeping: steadier nutrient delivery, reduced inflammation, and more effective clearance of toxic proteins. That combination could slow disease progression and boost the impact of other treatments.”
As this exciting research progresses, experts remain hopeful about its implications for managing and potentially reversing the effects of Alzheimer’s disease. If you have insights or a story tip related to health that Newsweek should consider, reach out via health@newsweek.com.